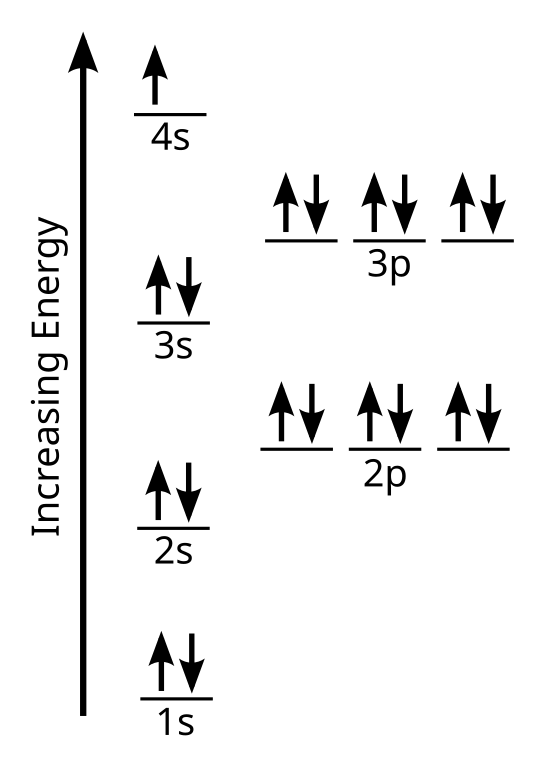
Level are called 'valence electrons.' These are the most important electrons when atoms bond. Valence Electrons 35 Note all elements in a Group have same number of valence electrons. Group 1: Li, Na, K, Rb, Cs, Fr Group 16: O, S, Se, Te, Po. Excited State 36 (e-have absorbed energy to. Valence electron, any of the fundamental negatively charged particles in the outermost region of atoms that enters into the formation of chemical bonds.Whatever the type of chemical bond (ionic, covalent, metallic) between atoms, changes in the atomic structure are restricted to the outermost, or valence, electrons.

Potassium Valence Electrons Gain Or Lose
The outermost shell of an atom is known as the valence shell. The electrons present in the valence shell of an atom are known as the valence electrons. The remainder of the atom—the nucleus and other electrons— is called the core of the atom. Electrons present in the core of an atom are known as core electrons. For example, the electronic configuration of sodium (Na) atom is
Na (11) = 2(K) 8(L) 1(M)
Thus, valence electrons in Na atom = 1
core electrons in Na atom = 2 + 8 = 10.
Potassium Valence Electrons

Image Source: surfguppy.com/wp-content/uploads/2014/03/780x1009xBONDS-Valence-electrons.jpg.pagespeed.ic.ld-82CrugW.jpg
Significance of valence electrons
1. The valence electrons of an atom are responsible for, and take part in chemical changes.
2. The valence electrons in an atom determine the mode of chemical combination, i.e., whether the atom in combining with other atoms can form ionic or covalent bonds is determined by the number of valence electrons present in the atom.
3. The valence electrons determine the combining capacity or the valency of the atom. The number of electrons in an atom that actually take part in bond formation is known as the valency of the element. For example, in carbon atom, there are four valence electrons.
C(6) = 2(K) 4(L)
The carbon atom is capable of forming four bonds. Hence, the valency of carbon is 4.
4. If the outermost shell of an atom is completely filled, valency = 0. For example, the outermost shells of helium, neon, argon, krypton etc. are completely filled, hence the valency of these elements is zero.
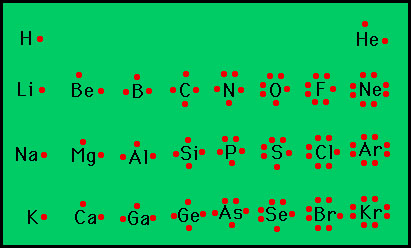
5. The elements having the same number of valence electrons in their atoms possess similar chemical properties. For example, all the alkali metals have one valence electron in their atoms. Hence, the chemical properties of the alkali metals are similar.

Potassium Valence Electrons Gain Or Lose
The outermost shell of an atom is known as the valence shell. The electrons present in the valence shell of an atom are known as the valence electrons. The remainder of the atom—the nucleus and other electrons— is called the core of the atom. Electrons present in the core of an atom are known as core electrons. For example, the electronic configuration of sodium (Na) atom is
Na (11) = 2(K) 8(L) 1(M)
Thus, valence electrons in Na atom = 1
core electrons in Na atom = 2 + 8 = 10.
Potassium Valence Electrons
Image Source: surfguppy.com/wp-content/uploads/2014/03/780x1009xBONDS-Valence-electrons.jpg.pagespeed.ic.ld-82CrugW.jpg
Significance of valence electrons
1. The valence electrons of an atom are responsible for, and take part in chemical changes.
2. The valence electrons in an atom determine the mode of chemical combination, i.e., whether the atom in combining with other atoms can form ionic or covalent bonds is determined by the number of valence electrons present in the atom.
3. The valence electrons determine the combining capacity or the valency of the atom. The number of electrons in an atom that actually take part in bond formation is known as the valency of the element. For example, in carbon atom, there are four valence electrons.
C(6) = 2(K) 4(L)
The carbon atom is capable of forming four bonds. Hence, the valency of carbon is 4.
4. If the outermost shell of an atom is completely filled, valency = 0. For example, the outermost shells of helium, neon, argon, krypton etc. are completely filled, hence the valency of these elements is zero.
5. The elements having the same number of valence electrons in their atoms possess similar chemical properties. For example, all the alkali metals have one valence electron in their atoms. Hence, the chemical properties of the alkali metals are similar.

